November 11, 2013
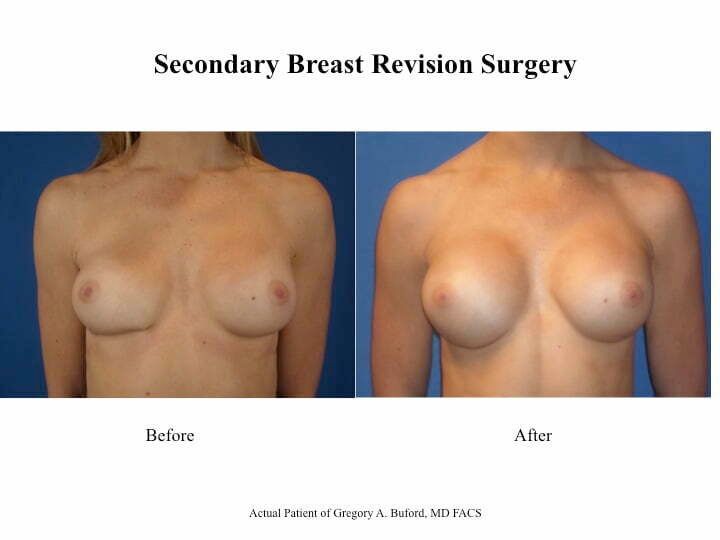
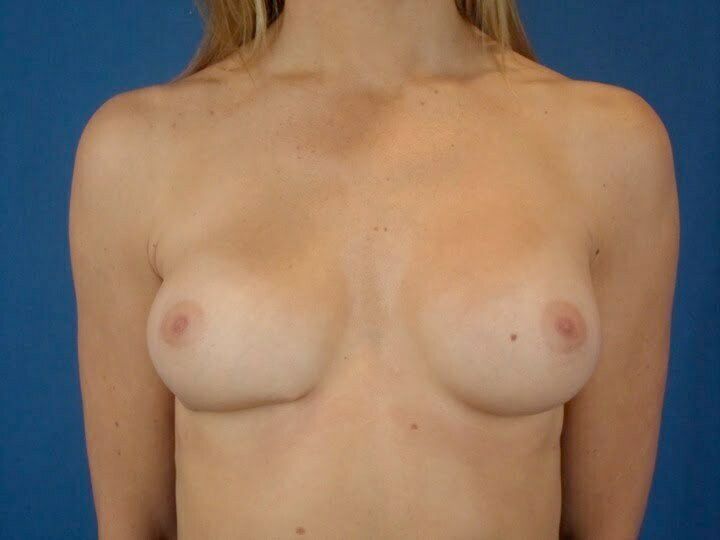
 I recently had the pleasure of seeing a young woman who had silicone breast implants placed by another Plastic Surgery but was simply not happy with the results. She initially had silicone gel implants under the muscle through an incision under her breasts but soon after her surgery noticed that one side had dropped. Her Plastic surgeon performed a procedure to tighten the scar tissue capsule on that side to try and elevate the implant but this procedure left her with puckering to both the lateral and medial edges and the result now looked even worse than it did before the procedure. When she saw me, she was frustrated about the appearance of her breasts and the relative asymmetry that had resulted and wanted to discuss her options. On examination, I noted that one side was puckered giving this breast a very distorted and unnatural look. And because the capsule had been tightened so much, there was distortion of the implant itself causing one edge to bulge out and creating a palpable point on her skin. My concern was that she not only had a sub-optimal result but also that over time this puckering would actually cause erosion of her overlying skin and eventually lead to exposure of the implant itself. She also expressed interest in a slight upsize to give her more fullness to the upper pole of her breasts. My recommendation was that we do the following:
I recently had the pleasure of seeing a young woman who had silicone breast implants placed by another Plastic Surgery but was simply not happy with the results. She initially had silicone gel implants under the muscle through an incision under her breasts but soon after her surgery noticed that one side had dropped. Her Plastic surgeon performed a procedure to tighten the scar tissue capsule on that side to try and elevate the implant but this procedure left her with puckering to both the lateral and medial edges and the result now looked even worse than it did before the procedure. When she saw me, she was frustrated about the appearance of her breasts and the relative asymmetry that had resulted and wanted to discuss her options. On examination, I noted that one side was puckered giving this breast a very distorted and unnatural look. And because the capsule had been tightened so much, there was distortion of the implant itself causing one edge to bulge out and creating a palpable point on her skin. My concern was that she not only had a sub-optimal result but also that over time this puckering would actually cause erosion of her overlying skin and eventually lead to exposure of the implant itself. She also expressed interest in a slight upsize to give her more fullness to the upper pole of her breasts. My recommendation was that we do the following:
- Replace both implants and modestly upsize
- Remove the previous capsular sutures to release the puckering
- Perform a secondary capsular tightening to more optimally position the breast fold
- Reinforce this repair with an ADM (acellular dermal matrix) graft of Strattice
 She ultimately underwent these procedures followed by taping of the fold and wearing of an underwire bra for 3 months. She has since been seen in follow-up and is extremely pleased with her results and the more natural appearance of her breasts. I will be posting similar cases in the coming months and look forward to your input. To learn more, please CLICK HERE to schedule your evaluation, call us at 30.747.6719, or email me directly at drbuford@beautybybuford.com Thanks again for your continued support. We look forward to hearing from you.
She ultimately underwent these procedures followed by taping of the fold and wearing of an underwire bra for 3 months. She has since been seen in follow-up and is extremely pleased with her results and the more natural appearance of her breasts. I will be posting similar cases in the coming months and look forward to your input. To learn more, please CLICK HERE to schedule your evaluation, call us at 30.747.6719, or email me directly at drbuford@beautybybuford.com Thanks again for your continued support. We look forward to hearing from you.