October 25, 2010
According to recent survey conducted by the American Society for Aesthetic Plastic Surgery (ASAPS), 40% of Plastic Surgeons in the United States are using silicone gel implants since their re-approval by the US Food and Drug Administration in 2006. Following some speculation regarding patient safety and the use of silicone, the FDA pulled all silicone breast implants off the market for further testing. However, after a 14-year moratorium and countless studies, many still ongoing, the FDA found silicone implants to be safe for use in breast augmentation patients 22 or older, and breast reconstruction patients of any age.
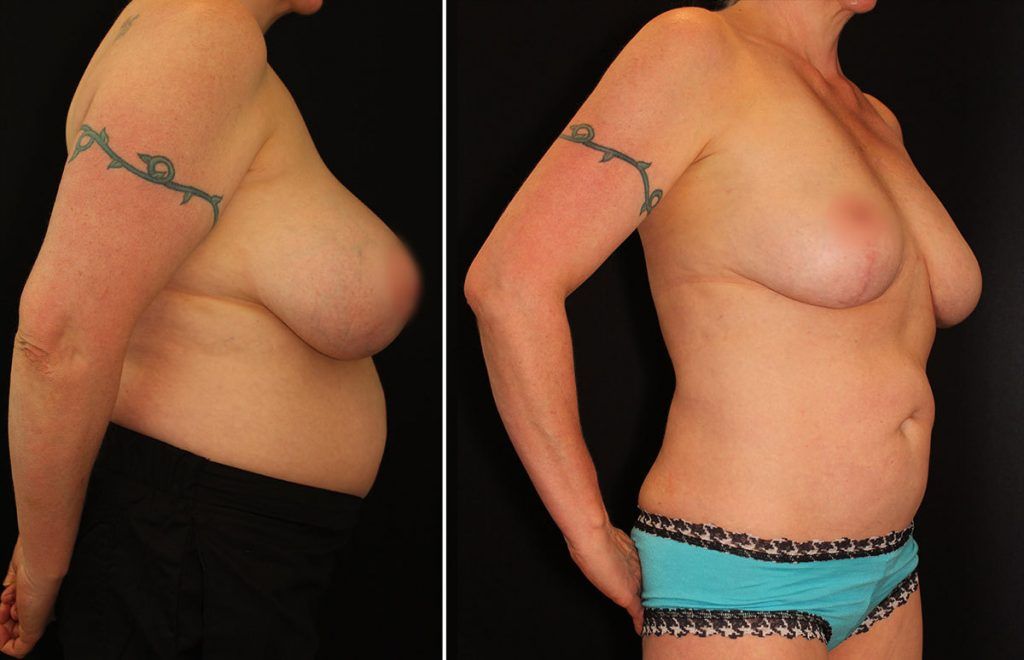
Dr. Buford says he believes silicone breast implants provide superior cosmetic enhancement results, mainly because the gel component closely resembles the look and feel of natural breast tissue. Safety and education are always top priorities, regardless of the type of implants his patients prefer. In the end however, Dr. Buford more closely aligns his professional expertise and opinion with the international preference for silicone gel breast implants.