October 27, 2015
At BEAUTY by BUFORD, we are always looking for ways to improve our results. Our patients are discerning and they are educated and they simply won’t accept a fairly good result. They want us to continually improve on our treatments and to look for ways to provide even greater and more long lasting benefits.
Introducing ThermiTox.
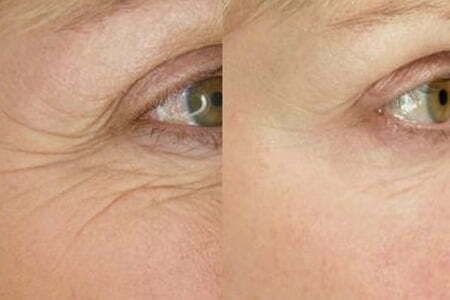
The revolutionary new ThermiTox procedure combines the proven results of BOTOX Cosmetic with the power of ThermiSmooth radiofrequency (RF) skin tightening and smoothing. Your first treatment will take no more than 45 minutes and will begin with ThermiSmooth to the skin around your eyes followed by gentle administration of BOTOX Cosmetic. From there, we suggest an addition 2 more ThermiSmooth treatments to the same area each spaced 2-3 weeks apart. Treatments are associated with ZERO downtime; we just ask that you not exercise or rub the treated areas for 6 hours following your initial treatment.
The combination of BOTOX Cosmetic and ThermiSmooth simply makes sense. Your treatment begins with a painless ThermiSmooth treatment, which heats the skin to encourage collagen remodeling and improvement of wrinkles. This is then followed by administration of BOTOX Cosmetic to help this skin in these treated areas tighten and rejuvenate under less tension so that maximum improvement can be achieved.
Standard pricing for this combination procedure will be: $500
- BOTOX Cosmetic (20 units)
- ThermiSmooth (3 treatments)
To introduce you to this amazing procedure, we are offering incentives on the ThermiTox procedure throughout the month of November. To learn more, please call us at 303.747.6719 or email us at Jen@beautybybuford.com
Thanks again for your continued support! We look forward to working with you.