January 30, 2016
One of the most challenging things to address in facial aging are those annoying lip lines that form as the result of not only smoking but also just the aging process itself. In the past, our go-to procedure for this area has been laser resurfacing. But while the results have been good, they have also been associated with several weeks of downtime which many of our patients simply aren’t willing to accept. Until now…
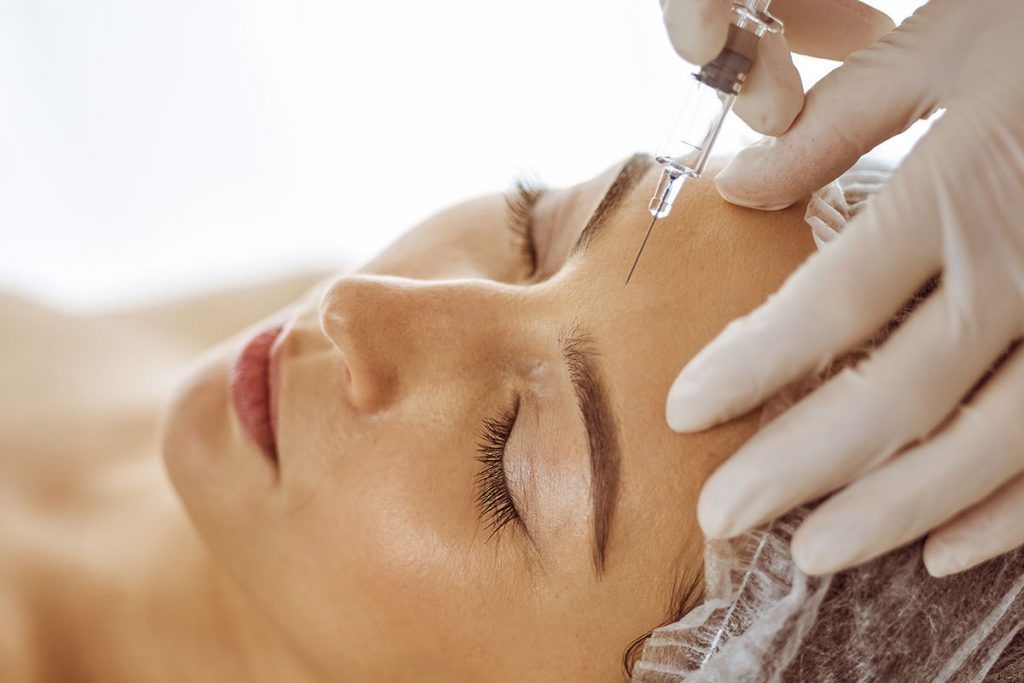
At BEAUTY by BUFORD, we are proud to announce the new ThermiFill procedure for lip rejuvenation. This innovative new technique combines the power of ThermiTight followed by administration of Juvederm Ultra XC to restore elasticity to this area and fill in those pesky wrinkles that, until now, have been challenging to address. The procedure begins with a 30 minute ThermiTight procedure performed in the office using locally instilled numbing solution. Through tiny openings on either side of the lip, the ThermiTight cannula is inserted into the white tissue above and below the lips and the radio frequency energy is then used to effectively stimulate collagen re-organization and re-alignment. Heat is administered in a precise and controlled manner to heat up the tissue and stimulate a local wounding response to which your body responds by stimulating growth of new collagen. The cannula is then removed and Juvederm Ultra XC is injected into the fine lines above and below your lips to plump out the wrinkles during this initial period of collagen stimulation. This secondary procedure is intended to provide temporary volume which will then bridge the gap between the initial time of treatment and the time to collagen stimulus when your body will effectively replace this temporary product with your own volume matrix.
For those patients susceptible to peri-oral cold sores, prophylactic medicine will be administered in addition to a light pain medication, as needed.
We are excited to introduce you to this innovative new procedure and look forward to your questions, comments, and insights. To see if you would be a good candidate, we encourage you to email a photo of your lips to melanie@beautybybuford.com. We will review your photo and let you know if we feel that this procedure would be effective for you! For any questions, we encourage you to call us at 303.747.6719.
Thank you again for your continued support. We look forward to working with you!